At least a couple times a month, customers ask us: “Why are there no white anodized aluminum jump rings?” The simple answer is that it’s not possible. (Scroll to the last paragraph to learn what “white” aluminum is.)
It seems like it should be possible because coloring aluminum is done by dye, and there are white dyes. However, these dyes cannot be used in anodized aluminum. When Jen and I visited our anodizer, he walked us through the process, and explained why this is so.
First, let’s look at what anodizing does to the surface of jump rings. The electricity in the anodizing process makes the metal porous, thereby preparing it to receive dye. Below are two images zooming in on the surface of the anodized metal.
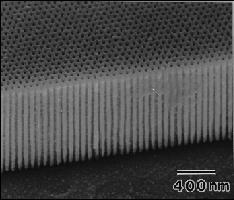

To be more specific, anodizing creates an oxide layer that has roughly one million microscopic pores on the surface of one square inch of aluminum. If you’d like to get your nerd on with more technical information about this process, SubsTech has lots of great articles (open-source!) on their website. The image below, from their page on anodizing, shows how the color is added to “fill the holes” in the surface of anodized aluminum.
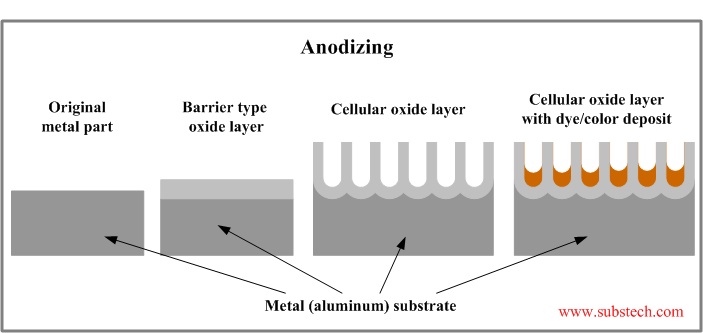
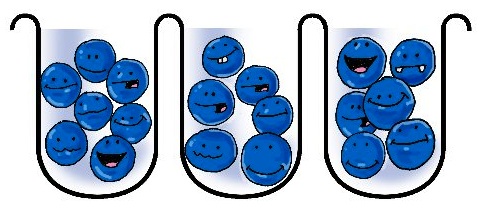
Now that you know a little more about the technical side of anodizing, let’s get back to our original question. It’s important to understand that not all dye molecules are created equal, and some colors are bigger than others. Blue molecules have no problem filling in the holes.
Red molecules are bigger than the blue ones, but they still fit, no problem.
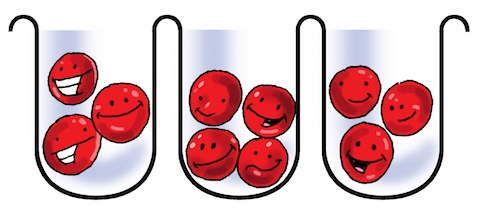
Unfortunately, when we get to white molecules, they are so large that they do not fit into the holes created by the anodizing process! There’s no way to squeeze a white molecule into the pore!
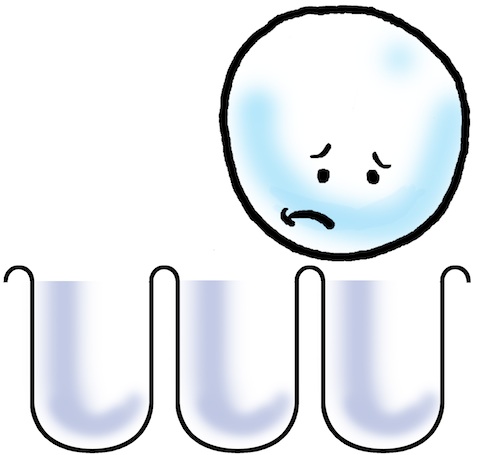
Another way to look at the size comparison of molecules:
Imagine a pore the size of a basketball net.
A gold dye molecule is the size of a golf ball.
Blue dye is about the size of a tennis ball.
Red and black dyes are about the size of a baseball.
Scientists haven’t been able to get white dye molecules smaller than beach ball size, which is too large to fit.
And that, fellow maillers, is why there is no white anodized aluminum.
Now, it’s true that a sort of white aluminum exists, but that color is not achieved by anodizing. You can get a color very close to white, by etching the aluminum and then sealing the surface just as would be done after dyeing colored anodized aluminum. The color is not a pure white, and can vary from a muted grey to a bright off-white. If you see someone selling “white” aluminum, they have most likely used this acid-etched process.



This is an all-time great article in our field and of course it was published by BBB! Your work IS timeless. 😉